Poor Blood Flow Causes Symptoms
When you stand, gravity pulls blood to your legs, briefly reducing blood flow to your brain. This is why we all sometimes get dizzy when we get up quickly. In healthy people, blood vessels constrict to squeeze the blood back up to the heart and brain. So that dizziness subsides within 15 seconds. In many medical conditions, this mechanism fails, keeping blood flow to the brain abnormally low, so the dizziness remains. This has been demonstrated on thousands of patients using ultrasound in specialized labs.¹,²
Poor Blood Flow Causes Symptoms
When you stand, gravity pulls blood to your legs, briefly reducing blood flow to your brain. This is why we all sometimes get dizzy when we get up quickly. In healthy people, blood vessels constrict to squeeze the blood back up to the heart and brain. So that dizziness subsides within 15 seconds. In many medical conditions, this mechanism fails, keeping blood flow to the brain abnormally low, so the dizziness remains. This has been demonstrated on thousands of patients using ultrasound in specialized labs.¹,²


This could only be measured in a handful of specialized labs, until now
Upon standing, Cerebral Blood Flow drops abnormally in people with Orthostatic Conditions, causing dizziness, lightheadedness, fatigue, and fainting symptoms
About Us
Seeing the Invisible: The Science Behind Lumia
Contents
Why Blood Flow
HR/BP Limitations
How Lumia Works
Scientific Validation
Collaborations
Awareness & Advocacy
Crowdsourced Science
NOTE: This page discusses forward-looking research and diagnostic-use type data related to Lumia. Diagnostic-type insights are not part of the wellness version of Lumia and have not been reviewed or approved by the FDA.
Why Blood Flow
Blood Flow Drops Cause Symptoms
When you stand, gravity pulls blood to your legs, briefly reducing blood flow to your brain. This is why we all sometimes get lightheaded when we get up quickly. In healthy people, blood vessels constrict to squeeze the blood back up to the heart and brain. So that feeling goes away within 15 seconds. In many medical conditions, this mechanism fails, keeping blood flow to the brain abnormally low, so the lightheadedness remain.
Blood flow drops during standing affect a wide variety of conditions. These include but are not limited to Dysautonomia, Postural Orthostatic Tachycardia Syndrome (POTS), Orthostatic Hypotension, Post-Viral Syndrome, Long COVID, Chronic Fatigue Syndrome (ME/CFS), Syncope, Ehlers-Danlos Syndromes (EDS), Sjögren's Disease, Peripheral Neuropathy, Preload Failure, Orthostatic Cerebral Hypoperfusion Syndrome (OCHOs), and Hypocapnic Cerebral Hypoperfusion (HYCH).
In addition, many other chronic conditions experience blood flow issues not just caused by standing, such as Heart Failure, Sepsis, Multiple Sclerosis (MS), Parkinson's, Alzheimer's, dementias, Diabetic Neuropathies, Hypertension, Fibromyalgia, Migraines, and Chronic Kidney Disease.
The main reason Cerebral Blood Flow is reduced in orthostatic conditions is because the heart fails to pump out enough blood (aka "Cardiac Output"). In the below chart of a tilt table study in 534 POTS, Orthostatic Hypotension, and ME/CFS patients, you can see how the reduction in the amount of blood the heart pumps out (Cardiac Output) has an almost 1:1 match with the Cerebral Blood Flow reduction.
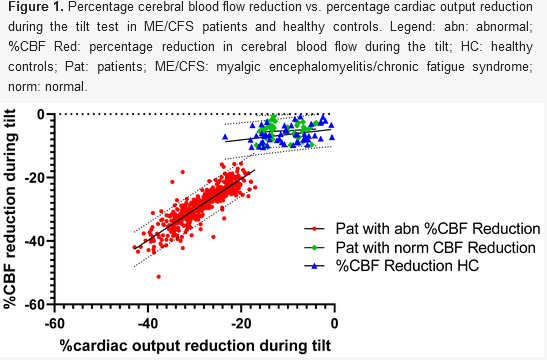
The primary reason the heart fails to pump out enough blood in these conditions is because not enough blood fills the heart before it pumps (aka "cardiac preload"). Squeezing an empty sack isn't very effective, so the heart's occasional attempt to compensate by increasing heart rate (i.e. POTS) is often futile.
Now the root cause for why not enough blood fills the heart varies widely across different diagnoses. Battling gravity to get blood up to the brain is a challenging task requiring coordination of multiple different systems. It takes only one system to fail for not enough blood to fill the heart. Common root causes include but are not limited to:
Reduced blood volume in the body (dehydration, hypovolemia, kidney disorder, endocrine disorders, etc)
Damage to peripheral nerves that tell veins to squeeze blood back to the heart (peripheral neuropathy, autonomic neuropathy, small fiber neuropathy, diabetic neuropathy, etc)
Damage to the central nervous system that regulates autonomic tasks like blood pressure management (Parkinson's, Alzheimer's, dementia, MSA, MS, nOH, PAF)
Connective tissue disorders where extra squishy blood vessels make it hard to squeeze blood back up to the heart (Ehlers-Danlos Syndrome, Hypermobility, etc)
Allergy or Immunology disorders where inappropriate and persistent systemic vasodilation make it hard to squeeze blood back to the heart (MCAS, mastocytosis, etc)
Venous obstructions that prevent blood return to the heart (May Thurner Syndrome, Nutcracker Syndrome, Pelvic Congestion Syndrome, etc)
Regardless of the root cause, each results in not enough blood returning to the heart which causes reduced blood flow to the brain. So increasing blood return to the heart is the primary focus to reduce symptoms.
In addition to the heart's failure to pump out enough blood, the blood vessels in the brain can incorrectly vasoconstrict and further reduce Cerebral Blood Flow, such as hyperventilation induced cerebral vasoconstriction. However, these are secondary effects that have been shown to come after the reduction in blood flow.²,³ While there are other factors causing reduced blood flow to the the brain, not enough blood returning to and filling the heart is the primary driver in reduced cerebral blood flow in orthostatic conditions.
Cardiologists and Neurologists know in theory that cerebral hypoperfusion (reduced blood flow) cause orthostatic symptoms, but it's hard to measure empirically.
Despite this measurement difficulty, cerebral hypoperfusion drives most treatment guidelines. First line treatment guidelines target increasing blood flow by increasing blood volume with extra fluid intake and electrolytes, and increasing blood return to the heart using compression garments.¹ First-line medications often target increasing blood volume or increasing vasoconstriction to increase how much blood fills the heart.
In addition to the treatment paradigm targeting increased blood flow, multiple studies have empirically demonstrated that drops in blood flow cause symptoms. In this 429 patient tilt table study, Cerebral Blood Flow reductions upon tilting correlated with increased symptoms upon tilting.
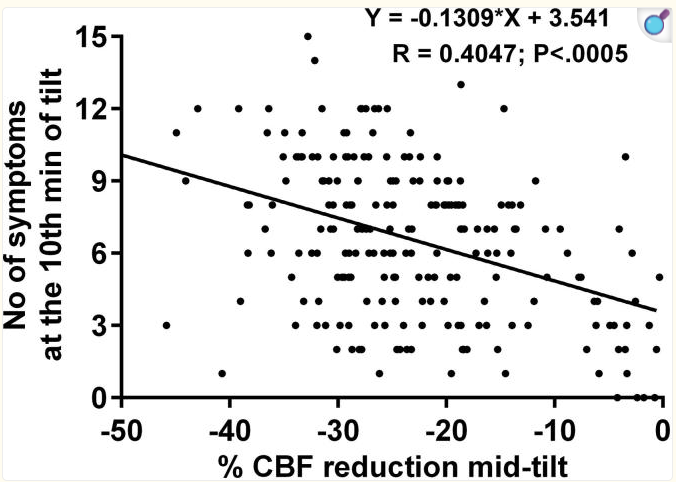
At Lumia, we reproduced similar results, where we also saw drops in Lumia's eCBF (External Carotid Blood Flow) metric correlate with symptoms with a significant Pearson r=0.46 correlation. Note that Lumia's eCBF is a future diagnostic output that is different than the Lumia Flow Index, and not currently available in the consumer version of Lumia for regulatory reasons.
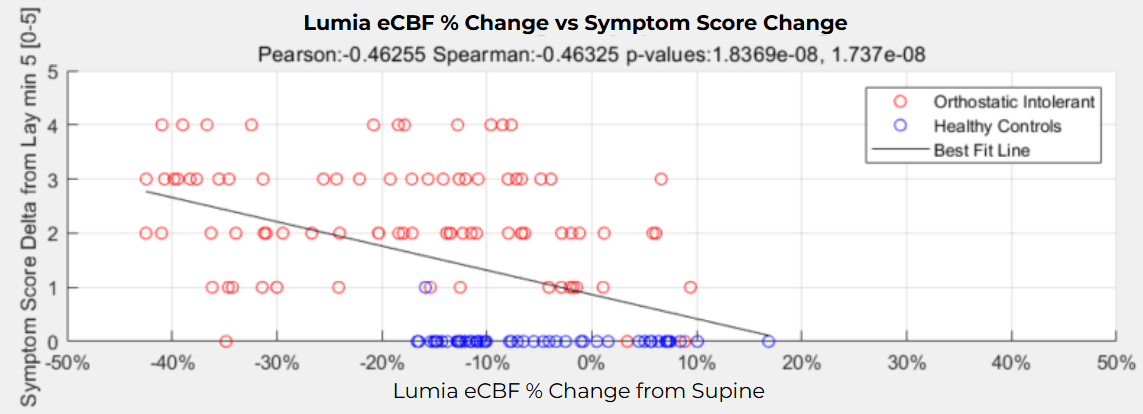
Since correlation is not always a great indicator of causation, this study looked at 71 patients who had come into a tilt table lab multiple times and observed that if Orthostatic Cerebral Blood Flow reductions worsened between clinic visits, patients' symptoms also worsened using a ROC classification statistical analysis, demonstrating a much stronger case for causation.
HR/BP Limitations
HR/BP Limitations
Heart Rate and Blood Pressure Frequently Miss The Picture
Doctors are taught to look for abnormal changes in Heart Rate (HR) and Blood Pressure (BP) upon standing. While this sometimes works for people who have POTS or Orthostatic Hypotension (though it lacks repeatability), most other chronic blood flow disorders have normal HR and BP despite significant symptoms, rendering these symptoms invisible. This misleads many doctors to conclude that nothing is objectively wrong.
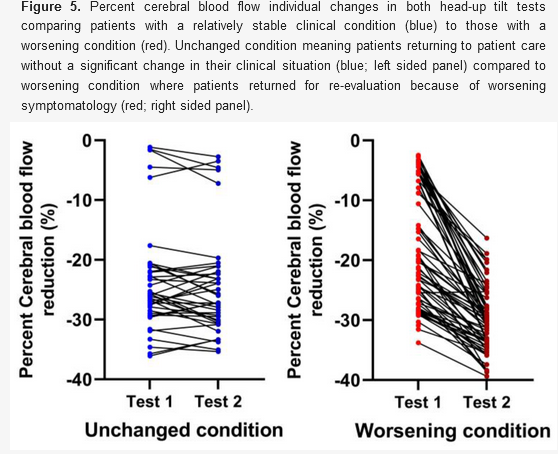
Measuring Cerebral Blood Flow reveals what's really going on, as has been proven on thousands of patients using specialized ultrasound techniques (See 1 and 2). For example, in the 429 patient study below, more than 90% of POTS (light blue bars), Orthostatic Hypotension (orange bars), and ME/CFS (dark blue bars) patients had >20% drops in Cerebral Blood Flow during tilt table testing, compared to Healthy Controls (HC) who only experienced a 7% CBF drop (red bars). In that study, 58% of patients with abnormal Cerebral Blood Flow had normal HR and BP.

It varies in different patient populations, but HR and BP can look normal in somewhere between 22% and 86% of patients despite orthostatic symptoms and abnormal Cerebral Blood Flow drops. Below are percentages of patients who exhibited normal HR and BP despite orthostatic intolerance:
22% of 744 patients during tilt table testing for orthostatic intolerance.
58% of 429 patients with orthostatic intolerance in ME/CFS despite low Cerebral Blood Flow
85% of 277 Long COVID patients during NASA Lean Test
29% of 29 Long COVID patients during tilt table testing when tested within 1 year of symptom onset, and 75% of patients when tested after 2 years of symptom onset.
86% of 50 Long COVID patients during standard autonomic testing.
67% of 24 Long COVID patients during tilt table testing.
Blood flow drop thresholds vary across studies due to differences in measurement techniques. Most methods provide proxy measurements rather than direct cerebral blood flow (CBF).
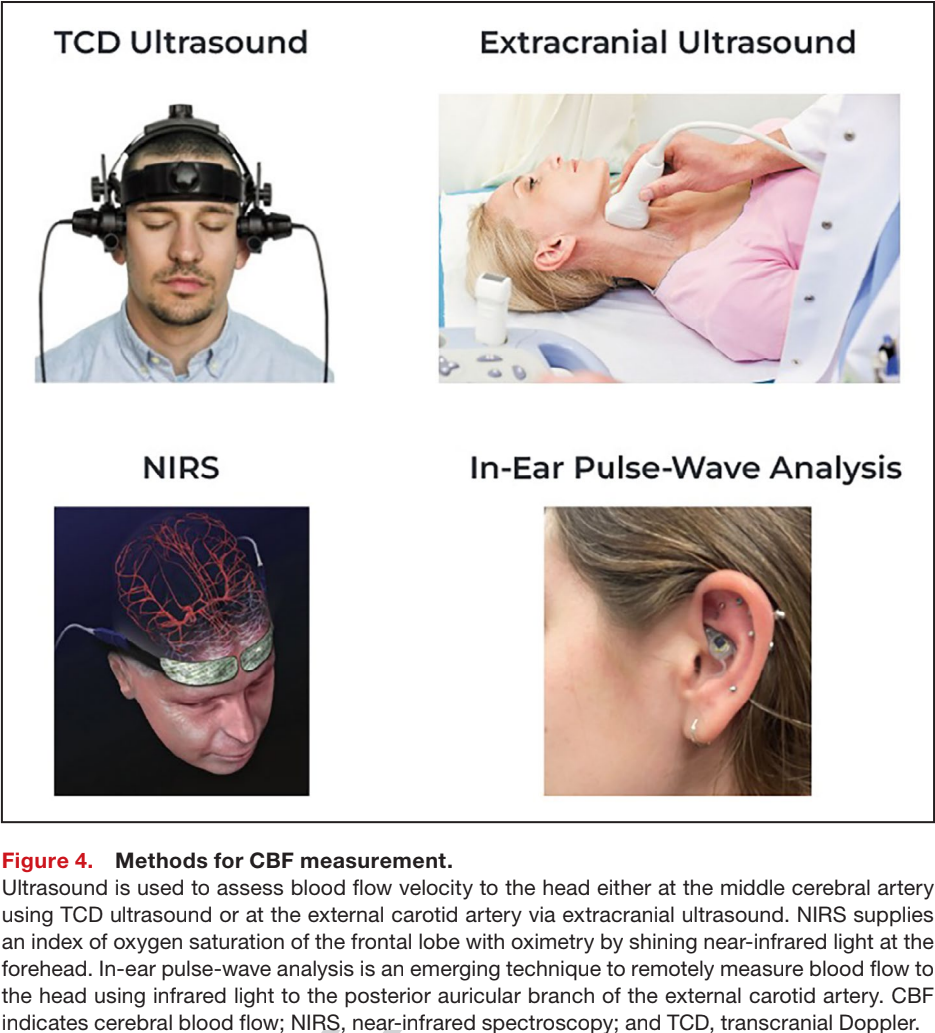
For TCD Ultrasound (measuring blood flow velocity in the Middle Cerebral Artery):
102 OCHOs: 24.1% ± 8.2% drop (vs. 4.2% ± 5.6% in controls)
9 POTS: 19.5% ± 2.6% drop (vs. 10.3% ± 2.0% in controls)
9 Long COVID: 20.0% ± 13.4% drop (vs. 3% ± 7.5% in controls)
For Extracranial Ultrasound (measuring total cerebral volume flow):
534 ME/CFS, POTS, and Orthostatic Hypotension: 29% ± 6% drop (vs. 6% ± 3% in controls)
So a 20%+ drop is likely abnormal, while 10% or less is likely normal. However, results depend on the measurement method and abnormal thresholds should be validated per technique.
Lumia’s Flow Index differs from ultrasound-based methods and cannot be back-calculated to these percentages to avoid misuse as a diagnostic. It is designed for personal insights, not diagnosis. It is scaled to a more understandable 0-100 index to be useful for daily use. This scaling factor alters the ability to calculate relative % changes in a way that can be compared to the >20% is abnormal, <10% is normal threshold above.
However, Lumia is actively undergoing scientific validation for future FDA diagnostic approval, at which time a calibrated % change will be provided.
The body has tools specifically designed to measure and regulate blood pressure. Pressure sensors (aka baroreceptors) in the heart and neck sense if blood pressure drops, causing your body to increase heart rate and squeeze blood vessels (aka increase Systemic Vascular Resistance) to try to raise the blood pressure.
However, while squeezing blood vessels helps raise the blood pressure in the center of your body, it further reduces blood flow. It's like placing your thumb on a water hose to spray water further - you can increase pressure to shoot water a further distance, but less water is flowing out of the hose overall.
This is why drops in blood flow often do not show up in blood pressure - your body maintains blood pressure by squeezing blood vessels, at the expense of blood flow. Unfortunately, blood flow is what your brain needs to function - it requires 20% of total blood flow as the most flow-dependent organ in the body. However, like in clinical medicine, blood pressure is used because it's what was possible to measure, not because it's the right thing to measure.
Now the body can squeeze specific blood vessels differently to attempt to selectively redirect more blood flow to certain organs. It's true that the brain can expand its blood vessels to allow more blood flow (aka "Cerebral Autoregulation") while continuing to squeeze other blood vessels. However, the blood vessels can only expand so much, and Cerebral Autoregulation eventually fails. As seen in the graph below of 534 patients and 49 healthy controls, Cerebral Autoregulation works when blood flow out of the heart (Cardiac output) drops less than 20% as Cerebral Blood Flow drops less than 10% - cerebral blood vessel dilation can somewhat mitigate the effect of reduced blood flow out of the heart. However, if the blood flow out of the heart starts dropping more than 20%, Cerebral Autoregulation hits its limits and CBF drops almost linearly with the reduction in Cardiac Output.

The divergence of blood pressure and blood flow is further complicated by the laws of physics - blood pressure at your arm as is measured in medicine does not reflect blood pressure at your head due to gravity. When you stand up, blood pressure at your head level is more than 20mmHg lower than at your arm level. In fact, standing head blood pressure could be 90mmHg/50mmHg while foot blood pressure could be 210mmHg/170mmHg. Gravity can be a stronger force than blood vessel autoregulation. This is an important law of physics that is often overlooked, and is especially relevant in conditions of orthostatic intolerance.
This issue of blood pressure and blood flow not matching up also shows up in non-orthostatic conditions as well, such as heart failure and hemorrhage.
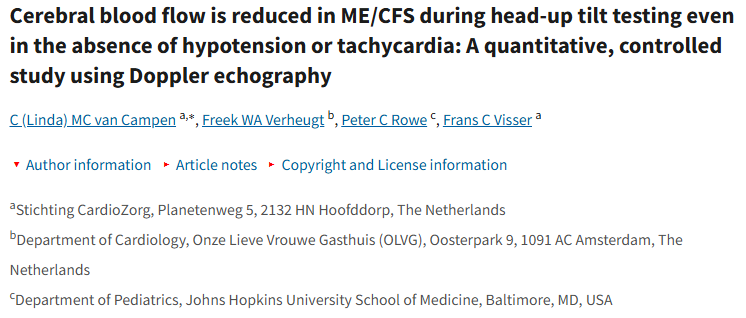
Below is a simulated hemorrhage where blood flow out of the heart drops (green line), blood flow to the brain drops (blue and orange line), but blood pressure stays constant (black "MAP" line) because of reactive blood vessel squeezing. The body's blood pressure auto-regulation often hides the fact that there's something wrong with blood flow. Blood Pressure does not equal Blood Flow.
How Lumia Works
How Lumia Works
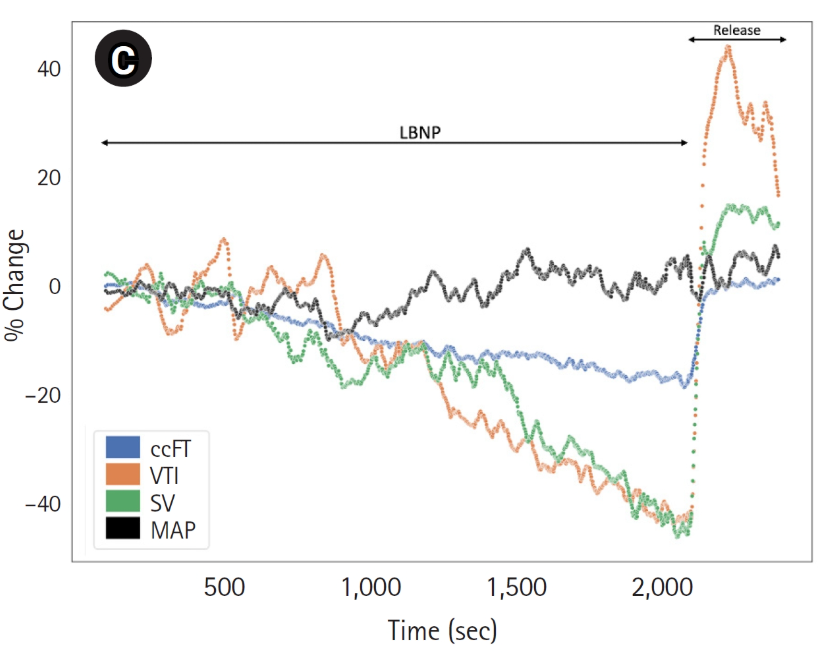
A Shallow Ear Artery: The Ideal Window to the Heart and Brain
Using the power of light, Lumia taps into a shallow ear artery to shine a light on what's really going on - drops in Blood Flow to the Head. NOTE: Lumia tracks "Blood Flow to the Head" and not "Cerebral Blood Flow" due to Lumia accessing an ear artery and not a brain artery.
Unlike most wearables that use green light to measure shallow capillaries that only carry basic heart rate signal reliably, Lumia uses infrared light on a shallow ear artery to capture an arterial blood flow signal. As a parallel branch to the Internal Carotid Artery that is at the same hydrostatic level as the brain, this ear artery is about as close to the brain as you can get non-invasively.
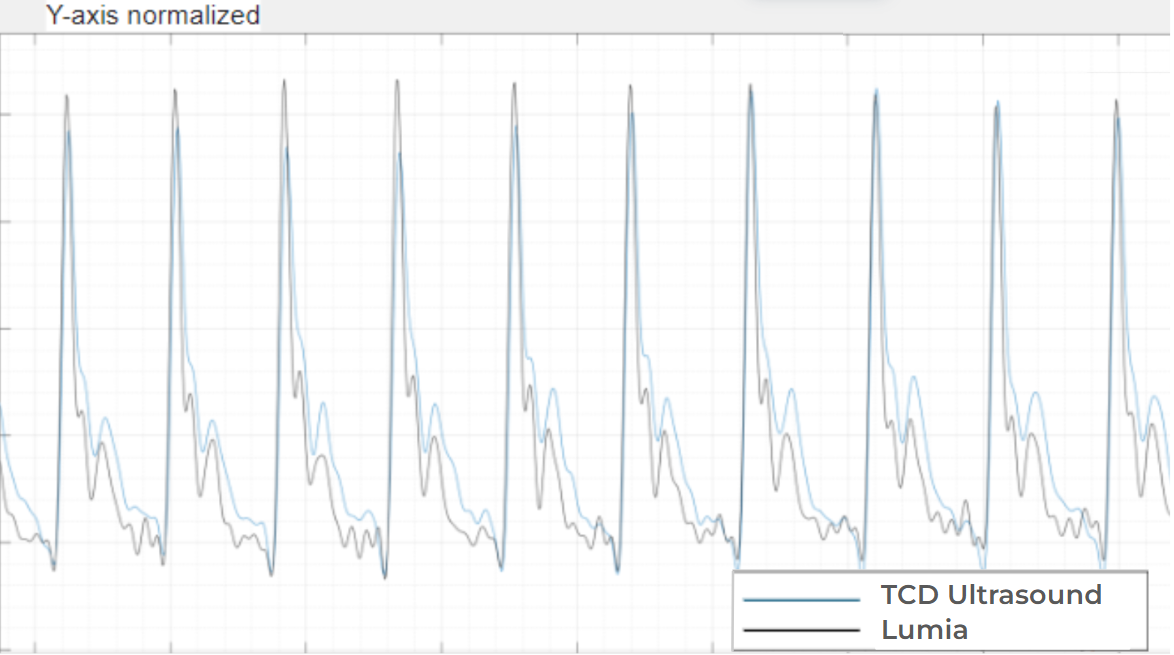
This shallow artery is what enables Lumia to capture an arterial blood flow signal that almost mirrors the blood flow waveform in the Middle Cerebral Artery as measured by TCD Ultrasound below:

Similar to how Continuous Glucose Monitors help diabetics track what matters, Lumia empowers those with chronic blood flow disorders to understand flow changes driving their symptoms.
Lumia taps into a branch of the Posterior Auricular Artery that climbs up the back of the ear and pokes through the ear cartilage to the front side of the ear in the Cymba Concha. Because it's so shallow (1-2 mm deep), Lumia is able to capture a strong arterial blood flow signal. This specific artery has been shown to be in that specific location in 16 out of 16 cadaver ears.
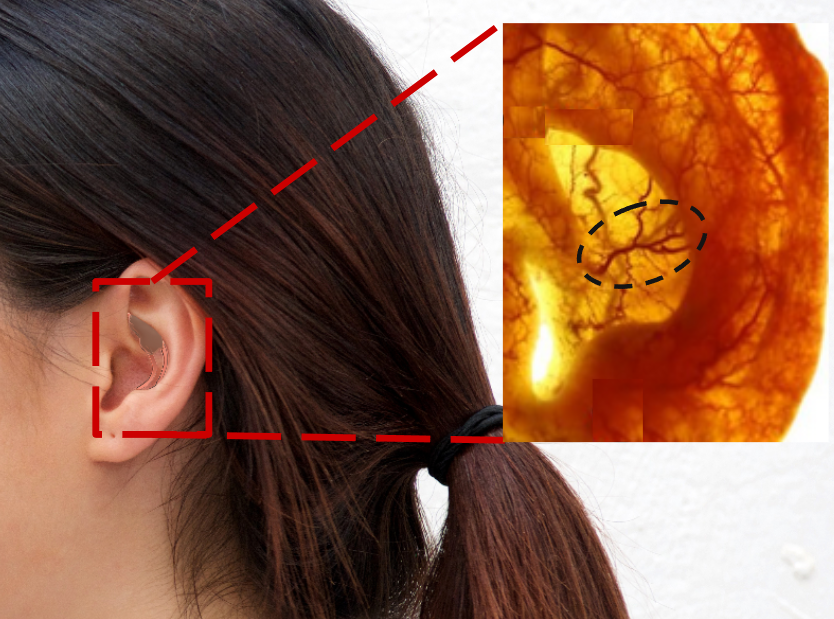
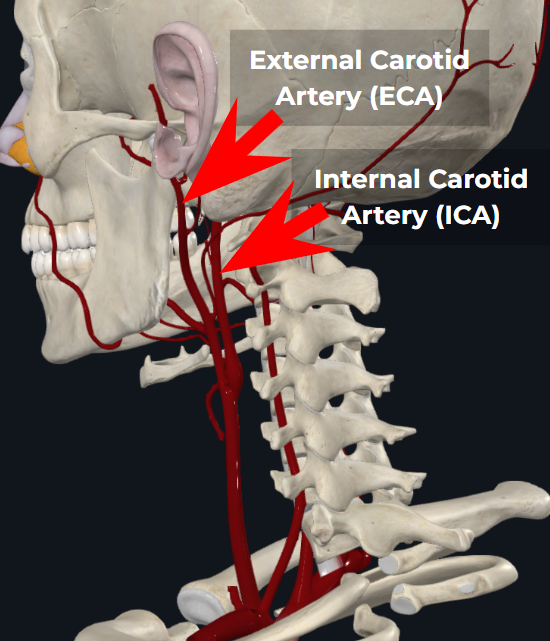
Yes, blood flow in the External Carotid Artery (ECA, which feeds the ear) and Internal Carotid Artery (ICA, which feeds the brain) can sometimes be different. This is particularly true in local ICA vasoconstriction situations, such as hyperventilation-induced hypocapnia. Or if there's a stroke in the ICA, which restricts flow in the ICA exclusively.
However in orthostatic conditions, reductions in Cerebral Blood Flow are primarily driven by a reduction in the amount of blood that the heart pumps out (aka "Cardiac Output").
Since orthostatic flow reductions happen upstream of both the ECA and ICA, the flow reductions show up in both arteries. As you can see in the anatomy depiction to the right, the ECA and ICA branch off the same artery, and the ECA at the ear is at the same hydrostatic height as the brain.
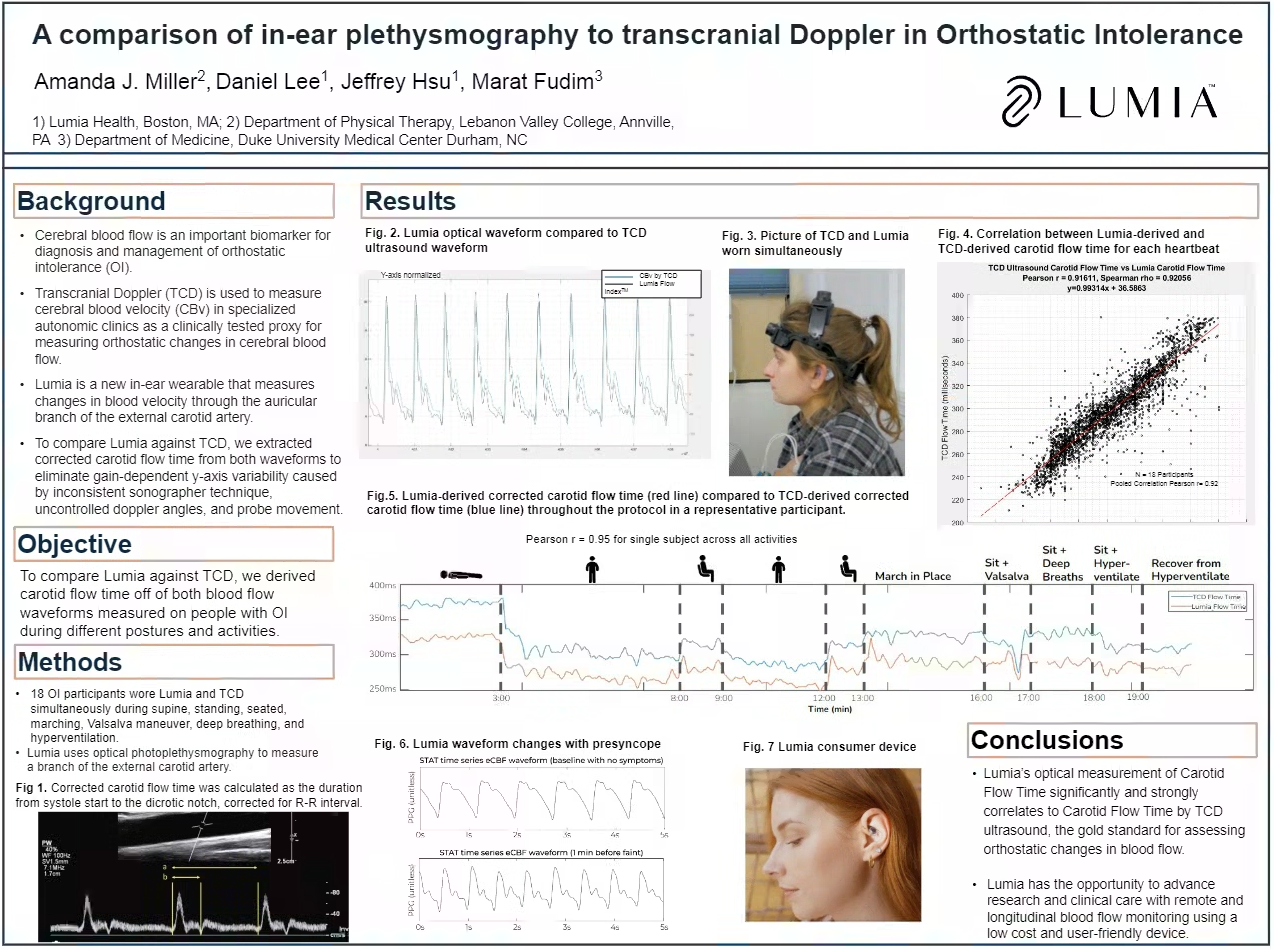
To further avoid factors that might affect the ECA and ICA differently, Lumia ignores the "y axis" or "amplitude" of the flow signal, which can be influenced by local ECA-specific factors such as temperature, CO2, vasopressors, etc. Instead, Lumia looks primarily at normalized morphological changes, such as how long the heart pumps for, which is driven by preload changes and is largely insensitive to local effects. As an example, "Corrected Carotid Flow Time," which has been clinically validated as a surrogate for stroke volume and gauging blood volume levels, is an important input feature into the Lumia Flow Index.
With the above novel physiology insights and algorithmic approaches, Lumia-derived Carotid Flow Time has been demonstrated to correlate with TCD Ultrasound derived Carotid Flow Time with a Pearson correlation r=0.91. So yes, ear blood flow changes track with brain blood flow changes when using a controlled physiological and algorithmic approach.
Scientific Validation
Tested against Standard Clinical References
Lumia's first clinical study was at Johns Hopkins during routine Tilt Table Testing, published in JACC. Lumia was able to measure blood flow abnormalites 2-12 minutes before patients passed out. In contrast, a standard clinical blood pressure cuff failed to take valid BP readings during 88% of dynamic fainting episodes.
With Duke collaborators, Lumia was then tested against simultaneous Transcranial Doppler Ultrasound during Stand Testing and Tilt Table Testing, demonstrating a remarkable r=0.91 Pearson correlation coefficient, comparing Lumia-derived Carotid Flow Time and ultrasound-derived Carotid Flow Time. The remarkable results of this multi-center study are currently being published to a high-impact peer-review journal, but preliminary results were presented at the American Autonomic Society (poster #115), and Dysautonomia International.
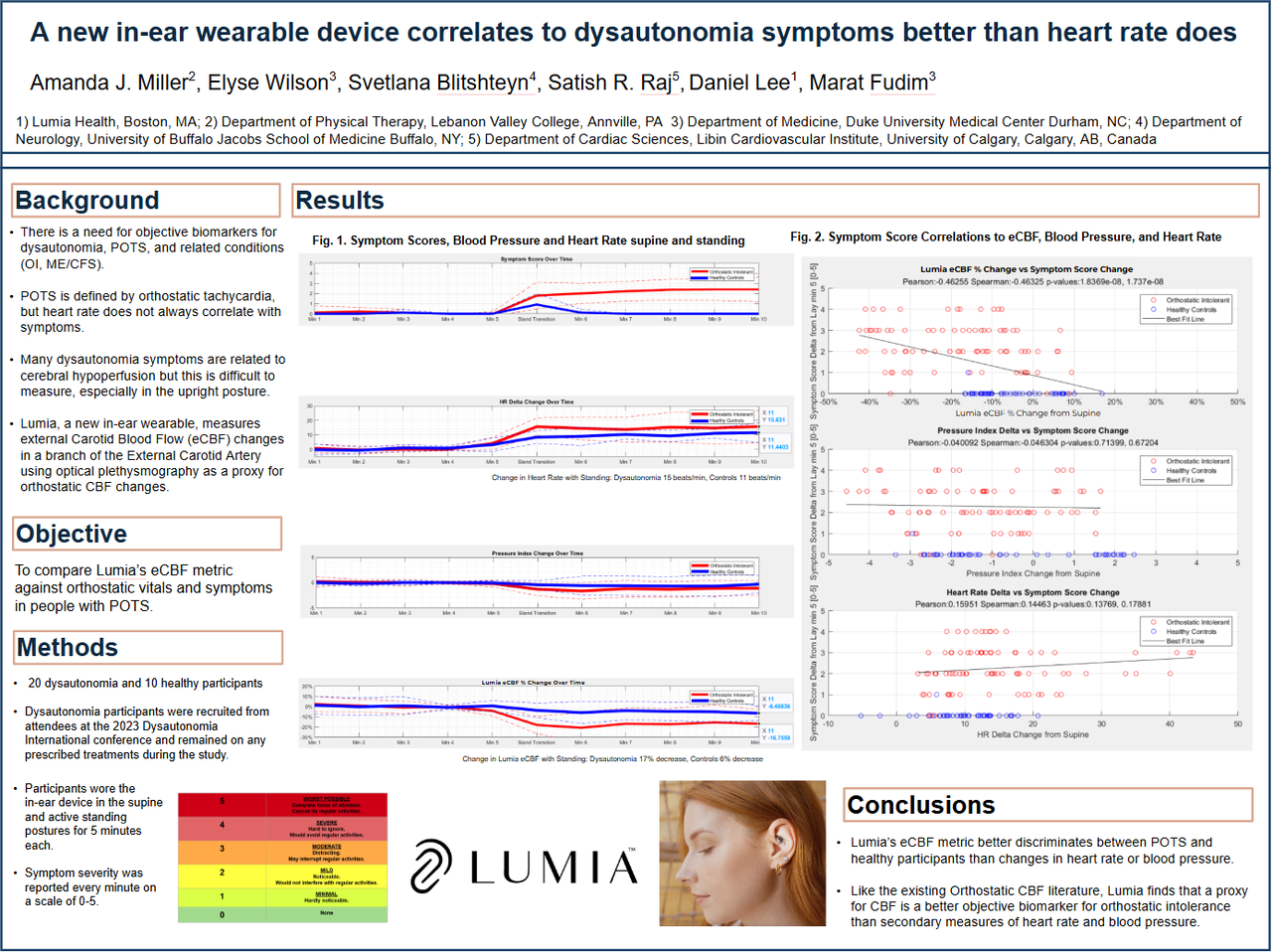
We also tested Lumia's ability to differentiate symptomatic orthostatic people from asymptomatic healthy controls during a 5 minute stand test. Lumia's eCBF metric (future diagnostic algorithm not currently available in consumer app) showed a 17% drop, compared to a 6% drop in healthy controls. In addition, Flow and patient-reported symptoms had a significant Pearson r=0.46 correlation coefficient (p<0.0001) compared to Heart Rate which had a non-significant correlation with symptoms (p=0.14).
This is just the tip of the iceberg of the research underway.

50
%
Off

Black Friday Sale
Whether you're looking for the perfect holiday gifts or just want to treat yourself, our Black Friday deals have something for everyone.
Collaborations
Collaborations
Collaborating with Scientific Luminaries
Lumia is providing a new telescope to advance the scientific understanding of many chronic blood flow disorders. The scientific community has responded powerfully with hundreds of prospective collaborators reaching out - we have been fortunate to collaborate with the best of the best.
If you are interested in using Lumia for a scientific study, connect with us here with details on your proposed study!
Partnered Institutions







Awareness and Advocacy
Awareness and Advocacy
Spreading the News About Blood Flow
We actively engage in scientific and medical discourse to show that chronic blood flow disorders are real—when measured correctly!
Our advocacy work doesn't stop at building the tools, but also raising awareness in the medical community about what and how to measure what matters. Through scientific publications, educational webinars, conference presentations, and direct engagement with physicians, we aim to amplify the science that a sensitive, accessible, and actionable biomarker does exist!
The science of blood flow and Lumia has thus far been published in the Journal of the American College of Cardiology (JACC), Journal of the American Heart Association (JAHA), American College of Sports Medicine (ACSM), and Clinical Autonomic Research (CAR). Lumia Health has also presented at the American Autonomic Society (AAS), Dysautonomia International, ACSM, and American Physical Therapy Association (APTA) conferences.
Recent publications
Research
Cerebral Blood Flow in Orthostatic Intolerance

Lumia's scientific collaborators conducted an exhaustive literature review, which reiterated the importance of measuring a proxy of Cerebral Blood Flow in order to increase sensitivity in detecting orthostatic abnormality.
Lumia Collaborators: Satish Raj (University of Calgary), Marat Fudim (Duke University), Hari Tandri (Vanderbilt), Amanda Miller (Lebanon Valley College)
Journal of the American Heart Association, Feb 2025
Read more
Research
Monitoring Carotid Blood Flow Using In-Ear Wearable Device During Tilt Table Testing

This research letter documented Lumia's first time on a Tilt Table Test (formerly known as STAT Health). Lumia's first scientific collaborator, Dr Hari Tandri of Johns Hopkins, placed Lumia on 20 patients undergoing routine orthostatic test evaluation and found Lumia saw abnormal reductions in blood flow 2-12 minutes prior to syncope episodes.
Lumia Collaborators: Hari Tandri (Johns Hopkins / Vanderbilt), Marat Fudim (Duke University)
Journal of the American College of Cardiology, May 2023
Read more
Crowdsourced Science
Data From the People, For the People
With our rapidly growing member community, we have the platform to warp-speed the science.
You can opt in to sharing your de-identified and aggregated data to an open-source research data pool, to grant researchers developing better diagnostics and therapeutics for these conditions to better understand what's really going on.
Rather than 30 person studies costing millions of dollars, Lumia allows
Lumia™ is designed for adults age 18 or over experiencing chronic symptoms that are made worse by standing, such as dizziness, brain fog, lightheadedness, fatigue, headaches, post-exertional malaise, and fainting. These are common in people with POTS, Syncope, Orthostatic Hypotension, Orthostatic Intolerance, Dysautonomia, as well as ME/CFS and Long Covid.
In these conditions, science has shown that blood flow to the brain drops during standing or sometimes even just sitting upright.
The Lumia™ program -- consisting of the Lumia™ wearable and app working together -- is the only one that can shine a light on blood flow changes to your head.
The Lumia™ wearable is worn only in the left ear, and is designed to be compatible with most, but not all, hearing devices and earbuds.
If you require the use of specific left ear devices, and aren't sure based on looking at photos of how Lumia sits in the ear, feel free to contact us with a photo of what you want to wear at the same time as Lumia.